SEMing the Inevitable
ø
After a restorative run and morning squash game with Mark, I spent the morning preparing a second set of SEM stubs of the diatoms I had sonicated and cleaned yesterday afternoon (the batch I’d made yesterday seemed a little too dense, having left behind a thick chalky white dot where the water dried up). I booked the next available SEM slot, which—miraculously—was at 1pm today, raced to get the stubs sputter coated, and checked them out.
The results are not far off what I expected to see—a lot of flimsy, warped diatoms—and also indicated that even the little sonicating I did yesterday (one blast of about 30 seconds or so, then three more short blasts of <5 seconds) was too much for these weakly silicified things, turning most of the material into rubble.
Here a selection of what I saw.
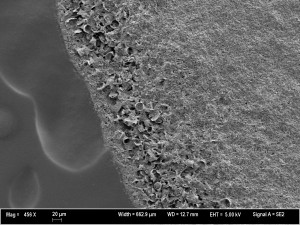
Each sample had a sharp edge where the water droplet was—with a lot of indiscriminate goop (organic matter?)/fine-grained debris over most of the surface, except at the edge, where some coarser material was visible:
There were some moderately intact valves amidst the debris:
Many of those looked like they were so flimsy they were essentially warping or folding; some had very large-pored/flimsy “skirts”:
The second set of samples I made were a little less crowded, but still far too dense:
Even the best-preserved specimens didn’t look like much:
For comparison, this is what the species I’m looking at here —Lithodesmium undulatum—is actually supposed to look like (courtesy of this website):